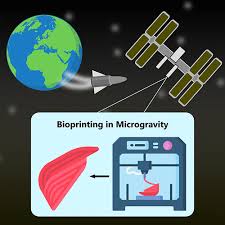
Executive Summary
3D bioprinting—layer-by-layer manufacturing of living tissues using cells, biomaterials, and bioactive molecules—has advanced swiftly on Earth, promising patient-specific tissue grafts, organ models for drug testing, and ultimately full organ replacement. Microgravity adds a new dimension: by removing the constant burden of gravity, soft tissues can be assembled without the structural constraints that limit Earth-based fabrication. Early space-based demonstrations on the International Space Station (ISS) have shown that bioprinters can deposit living cells in orbit and form structures that exhibit promising biology. This article explores the science behind why microgravity matters for bioprinting, the technical approaches, the state of experiments in low Earth orbit, medical and mission-oriented applications, engineering challenges, ethical and regulatory considerations, and a practical roadmap toward realizing organ production in space.
Why Bioprinting? The Medical Imperative
The shortage of transplantable organs is a persistent global health crisis. Thousands die annually waiting for suitable donor organs; many more live with chronic organ failure and diminished quality of life. Bioprinting aims to create functional tissues and organs built from a patient’s own cells, reducing rejection risk and eliminating long waitlists.
Beyond transplantation, bioprinted tissues accelerate drug discovery and precision medicine by providing human-relevant models for toxicity testing, disease modeling, and personalized treatment screening. The translation from millimeter-scale tissue constructs to clinically meaningful, vascularized organs remains the field’s central technical challenge.
What Microgravity Changes: The Biology and Physics
Gravity shapes biology at every scale. On Earth, cells sense and respond to mechanical cues provided by gravity, substrate stiffness, and hydrostatic pressure. For bioprinting, gravity imposes immediate practical constraints:
- Soft bioinks sag or slump during and after deposition, requiring rapid crosslinking or supporting scaffolds.
- Large, porous structures must be printed with internal supports or sacrificial materials to maintain shape.
- Cells sediment within viscous bioinks, causing inhomogeneities.
Microgravity relaxes these constraints. In microgravity:
- No sagging: Extruded filaments and droplets can retain delicate geometries without heavy structural scaffolding.
- Gentle assembly: Cells suspended in low-viscosity carriers can remain evenly distributed rather than settling out.
- Novel morphogenesis: Reduced mechanical stress and altered fluid behavior can change how cells self-organize, differentiate, and assemble extracellular matrix—potentially promoting complex tissue architectures that are difficult to reproduce on Earth.
At the same time, microgravity modifies cell signaling, cytoskeletal organization, and gene expression. These changes are not uniformly beneficial: some tissues may differentiate differently or mount unusual stress responses. Understanding these effects is part of the research agenda.
Bioprinting Technologies: How to Build Living Tissues in Space
Multiple bioprinting approaches have been adapted for microgravity. Each has trade-offs around resolution, cell viability, printing speed, and materials compatibility.
Extrusion-Based Bioprinting
The most common method uses pneumatic or mechanical extrusion of a cell-laden hydrogel (bioink) through a nozzle. Advantages include compatibility with high cell densities and a wide range of bioinks. In microgravity, extrusion benefits from low sagging, enabling suspended lattice-like structures and larger overhangs without support materials.
Inkjet and Droplet-Based Bioprinting
Droplet deposition technologies produce high-resolution patterns by ejecting picoliter-to-nanoliter droplets. In microgravity, droplet trajectories and coalescence behave differently; specialized ejection control and fluid handling strategies mitigate unwanted motion. These printers excel at patterning multiple cell types with micron-scale precision.
Laser-Assisted Bioprinting (LAB)
LAB uses focused laser pulses to transfer cell-laden material onto a substrate with high spatial resolution and minimal nozzle clogging. The contactless nature of LAB is appealing for space, but the optical complexity and power demands must be accommodated onboard.
Stereolithography and Photopolymerization
Stereolithographic approaches use light to selectively crosslink photosensitive bioresins, producing high-resolution structures. Microgravity allows the formation of delicate microchannels and freeform architectures without support. However, suitable biocompatible photopolymer systems need careful development.
Hybrid and Multi-Modal Systems
Combining methods lets engineers exploit the high resolution of droplet or laser systems with the volumetric throughput of extrusion printers, producing vascularized scaffolds seeded with multiple cell types.
Bioinks and Biomaterials for Space Bioprinting
Bioinks must balance printability, mechanical integrity, cellular compatibility, and long-term functionality. Common materials include natural hydrogels (alginate, gelatin methacryloyl—GelMA, collagen), synthetic polymers (PEG derivatives), and composite materials with reinforcing fibers or nanoparticles.
In microgravity, the reduced need for mechanical stiffness during printing opens the door to softer matrices that better mimic native extracellular environments. However, post-print stabilization—via enzymatic crosslinking, ionic gelation, or photo-crosslinking—remains critical to maintain construct shape during maturation, handling, and transportation back to Earth.
Advanced bioinks can contain growth factors, controlled-release particles, and printed microvasculature templates to encourage host-like tissue development. For clinical translation, bioinks must use GMP-grade components and be compatible with regulatory pathways.
Vascularization: The Core Technical Barrier
No organ can survive without an efficient blood supply. Building multi-centimeter tissues requires engineered vascular networks to provide oxygen and nutrients and remove waste. Techniques to achieve vascularization include:
- Sacrificial printing: Print a fugitive ink (e.g., Pluronic F127) as a template, surround it with bioink, then remove the sacrificial material to leave perfusable channels.
- Co-printing endothelial cells: Directly print endothelial cells in filamentous patterns that self-assemble into vessel-like structures.
- Angiogenic factor gradients: Embed growth-factor-loaded particles that recruit and guide vessel growth during maturation.
Microgravity helps by allowing delicate, branching sacrificial templates and complex channel geometries to be printed without collapse. Moreover, microgravity alters fluid perfusion during culture; perfusion bioreactors must be rethought to provide convective nutrient transport in the absence of buoyancy-driven flows.
Organoids and Self-Assembly: A Complement to Direct Printing
Organoids—self-organizing, stem-cell-derived mini-organs—are proving powerful for modeling development and disease. Bioprinting in microgravity can combine organoid biology with structural scaffolds: printed scaffolds can spatially organize organoids into larger-scale assemblies that fuse, mature, and integrate vasculature.
Microgravity itself influences organoid formation: studies show changes in organoid size, gene expression, and morphology, which could be harnessed to encourage more mature tissue phenotypes.
Space-Based Bioreactors, Maturation, and Life Support
Printing is only the first step—constructs require days-to-months of controlled culture to mature. Space-based bioreactors must supply controlled temperature, gas exchange, sterile fluid handling, and mechanical cues (stretch, flow) that mimic physiological conditions.
Innovations include:
- Microgravity-compatible perfusion systems that use positive displacement pumps and closed-loop microfluidic cartridges to circulate media without relying on gravity.
- Microenvironment control for oxygen tension and pH using integrated sensors and feedback control loops.
- Automated imaging and analytics for remote monitoring of tissue growth.
Crew time is limited; automation and remote operation are therefore critical.
Case Studies and Demonstrations on the ISS
The ISS has served as the proving ground for initial bioprinting experiments.
- BioFabrication Facility (BFF): Developed through partnerships (Techshot and nScrypt among them), the BFF was designed to enable 3D bioprinting with living cells aboard the ISS. Experiments have included printing meniscus tissue, heart cells, and other constructs, demonstrating that live cells can be printed and recovered for Earth-based analysis.
- 3D Bioprinting Solutions (Invitro) experiments: A Russian team conducted early demonstrations of space bioprinting that established key methods and logistical lessons for on-orbit operations.
- Commercial and academic payloads: Multiple university and commercial experiments have tested bioink behavior, cell viability, and organoid formation in microgravity.
These experiments validate core concepts—cell survival after printing, feasibility of complex geometries, and novel biological responses—but they remain preliminary. Scaling to organ-sized constructs with integrated vasculature is the next step.
Applications: From Deep Space Medicine to Earthly Therapies
Clinical Transplantation on Earth
Paradoxically, the ultimate prize—functional transplantable organs for humans—may first be manufactured in microgravity and then returned to Earth. Advantages include the ability to create large, minimally supported tissues with more native-like microarchitecture. However, reconditioning tissues for implantation after exposure to space (radiation, microgravity-modulated gene expression) will require careful validation.
On-Demand Wound Care and Regenerative Therapies in Space
For long-duration missions to the Moon or Mars, the ability to print skin, bone grafts, or cartilage on demand could be life-saving. Small bioprinters could be deployed on crewed missions to produce personalized grafts for trauma or chronic wounds.
Drug Discovery and Disease Modeling
Microgravity-grown tissues may exhibit different maturation pathways, offering unique models for cardiac, neural, a